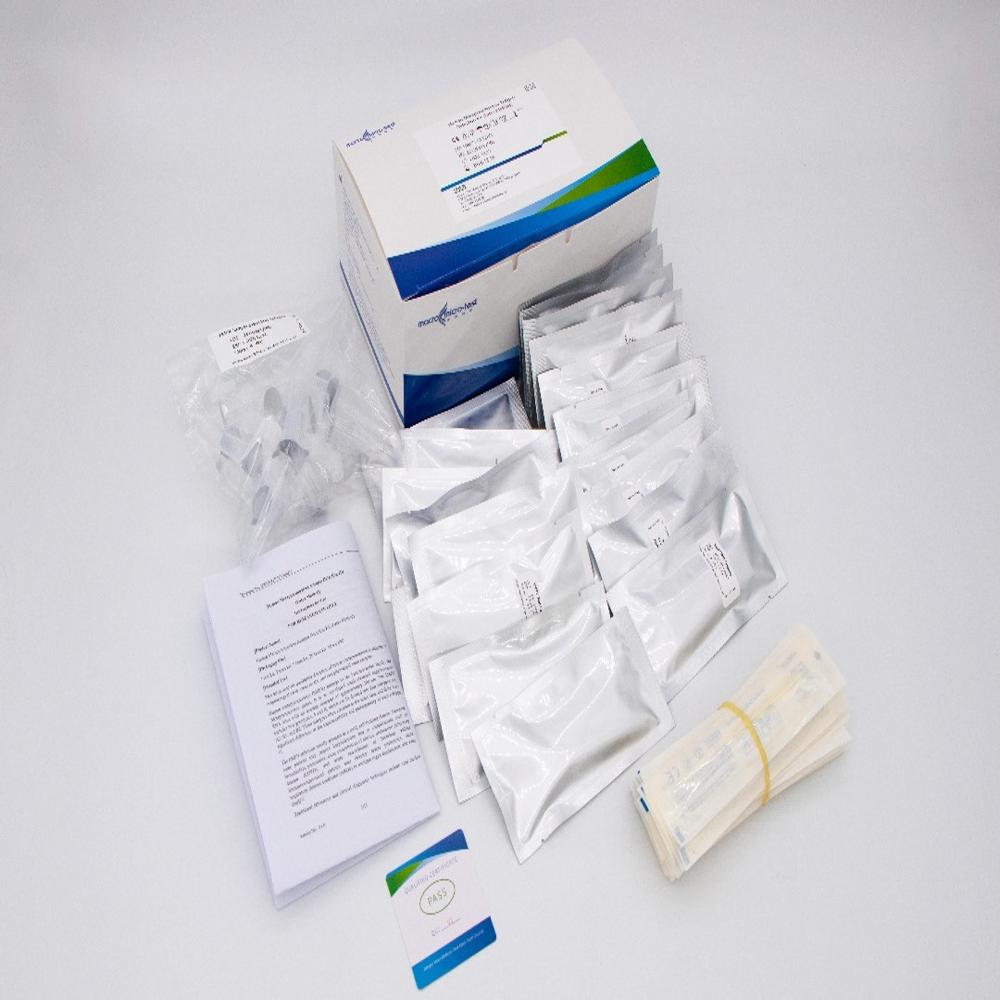

HMPV
550.0 INR/Piece
Product Details:
X
HMPV Price And Quantity
- 20 Piece
- 550.0 INR/Piece
HMPV Trade Information
- Cash Advance (CA)
- 1000 Piece Per Day
- 1 Week
- Yes
- Contact us for information regarding our sample policy
- 20 tests per Box Test Cassetes Sample Extraction Solution Nasal swab.
- Asia Australia Central America North America
- All India
- CE, IVD
Product Description
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email